Active Ingredient Prescribing
Prescriptions created in Communicare V20.2 and later meet the requirements of the Active Ingredient Prescribing legislation (2019), which is mandatory from 1 February 2021.
This legislation ensures that doctors make a clinical decision about the inclusion of the brand by prohibiting prescribing software from including brand names on prescriptions by default.
To meet the requirements of the legislation, set your Prescribing Options to Generic Prescribing. For more information, see Prescribing options in System Parameters - Clinical .
Prescriptions created before the introduction of active ingredient prescribing are displayed according to the new rules if doing so does not change the original intent of the prescriber.
For those medications on the LEMI, prescriptions created before the upgrade are displayed as intended by the original prescriber. However, if a prescription is reprinted, the format abides by the new rules for prescriptions.
Generic medications
Active ingredients are included on all Pharmaceutical Benefits Scheme (PBS) and Repatriation PBS (RPBS) prescriptions, except for medications with four or more active ingredients and a number of other specified items (see LEMI and LMBC below).
For generic medications, Communicare lists each of the items in the pack where each item is made up of one or more active ingredients with varying strengths.
active_ingredient1 strength, active_ingredient2 strength,
active_ingredient3 strength, active_ingredient4 strength
form unit_volume [pack_size] Rpts:number_of_repeats- PBS prescriptions - if the generic composition contains only one item with one active ingredient, the form is not included.
- However, if the formulation of the product contains the terms modified or release, each active ingredient in the product indicates the form.
- Also, if the formulation of the product does not contain the terms "modified" or "release", but an active ingredient within it does contain these terms, this active ingredient indicates the form of the ingredient.
- The volume information is added only when it is available. If the volume is 1 per unit, that is 1 / g, the 1 is ignored. For example, 50 mg / g compared to 50 mg / 2 g.
- If subpackage information is present, this is used, otherwise the number of items per pack is used. For example, [8]x2 means that there are 2 subpackages of 8 items in each pack.
Examples
- One item, with one active
ingredient:
Metformin hydrochloride 500 mg coated tablet - One item, with three active ingredients of varying
strengths:
Aluminium hydroxide 250 mg/5 mL, Magnesium hydroxide 120 mg/5 mL, Magnesium trisilicate 120 mg/5 mL oral suspension 500 mL [1]x2 - Two items, each with one active
ingredient:
Peginterferon alfa-2b 80 mcg powder for injection [4] & Ribavirin 200 mg capsule [140] - Two items, with three active ingredients
each:
Paracetamol 300 mg, Dextromethorphan hydrobromide monohydrate 10 mg capsule & Paracetamol 300 mg, Dextromethorphan hydrobromide monohydrate 10 mg, Doxylamine succinate 6.25 mg capsule [12]
Brand medications
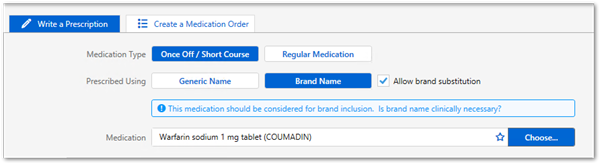
Prescribers may continue to include a brand name on prescriptions wherever clinically necessary for their patient.
active_ingredient strength form (BRAND_NAME)Warfarin sodium 1 mg tablet (COUMADIN)LEMI
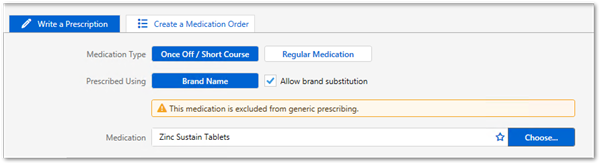
For those medications on the LEMI, prescriptions created before the upgrade are displayed as intended by the original prescriber. However, if a prescription is represcribed or reprinted, the format abides by the new rules for prescriptions, except for medications that are represcribed in bulk. For these medications, if they were prescribed by active ingredient before the upgrade and are on the LEMI, they are represcribed by active ingredient.
LMBC medications
Medications included in the LMBC are flagged in Communicare. Providers should consider prescribing these medications by brand. For example, Marevan and Coumadin are not bioequivalent despite both having the same active ingredient of warfarin sodium, so should be prescribed by brand.
Extemporaneous preparations
generic
recipeactive_ingredient strength form, volume (FREE TEXT)
recipeFREE
TEXT indicates that this is a custom medication.Boric acid 1g Solution, 60 mL (FREE TEXT)
Formulation: Mix 20mL of 5% Boric Acid solution with 40mL of deionised or distilled water.